-Strive towards a new high of 100 billion yen sales in FY 2020 (combined earnings, approx. 1.8 times FY 2014)
-Enter new field and generate global synergy through the purchase of Eidia Co., Ltd.,
-Establish a diagnostic reagent manufacturing and sales subsidiary in Suzhou, China
Sekisui Medical Co., Ltd. (President and Representative Director: Hideo Tagashira, hereinafter called "Sekisui Medical"), a 100% wholly-owned subsidiary of Sekisui Chemical Co., Ltd. (President and Representative Director: Teiji Koge, hereinafter called "Sekisui Chemical") has now established its 5-year midterm management strategy, "HIYAKU (Leap) 2020" (hereinafter called the "new midterm plan") aims to increase sales to 100 billion yen by FY 2020 (combined earnings, approx. 1.8 times FY 2014).
■Sekisui Medical Midterm Management Strategy "HIYAKU (Leap) 2020
1. Background
Sekisui Chemical has positioned the “Diagnostic System” as one of the 8 businesses key to the growth of the entire group (the “Growing 8”), and at its center Sekisui Medical will focus efforts on the expansion of the life science(medical) field which includes diagnostic reagent systems. In recent years we have expanded the scale and scope of our global business through the active implementation of M&A and other growth strategies.
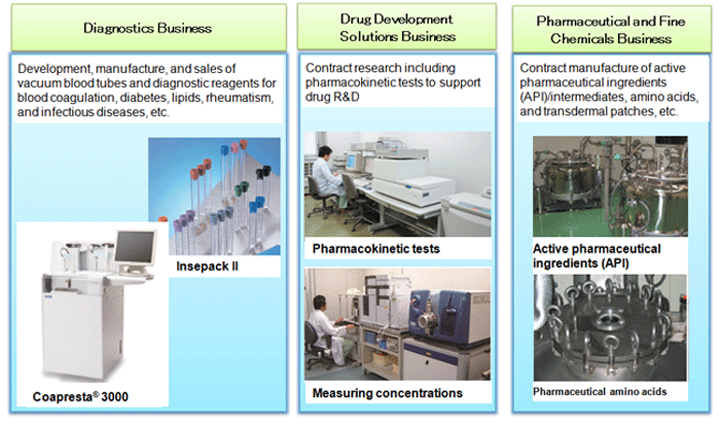
2. Sekisui Medical Business Overview
Sekisui Medical is developing three primary business operations; the Diagnostics Business centers on diagnostic reagents, the Drug Development Solutions Business supports Pharmaceutical companies with research and development, e.g. being contracted for pharmacokinetic testing, and Pharmaceutical and Fine Chemicals Business which is responsible for the contract manufacture of active pharmaceutical ingredients (API) etc for various pharmaceuticals.
|
|
|
<Reference>SEKISUI MEDICAL CO., LTD. |
3. Essential Strategy of the New Midterm Plan
Sekisui Medical cites "global one management" as its fundamental policy, and aims to establish global production, sales, and development, along with regulatory and quality assurance systems as well as promoting global consolidated management of all businesses and achieve net sales of 100 billion yen by FY 2020.
At the base of this fundamental policy, we will drive the new midterm plans through the twin strategies of strengthening core business and expanding frontiers.
1) Strengthening core business
Our plan is to strengthen profitability by positioning the following three items as core businesses: Diagnostics: Domestic, Diagnostics: Western, and Pharmaceutical.
(1) Diagnostic Reagents (Domestic)
In December last year Sekisui Chemical acquired all stocks of Eidia Co., Ltd., which manufactures and sells diagnostic reagents from Eisai Co., Ltd. We plan to expand our business operations with a renewed focus on oncology as a key area, opportune due to the strengths of Eidia in the field of oncology.
<The 6 Focus Areas and Main Products>
|
Field |
Clinical Chemistry/ |
Blood Coagulation |
Diabetes |
Infectious Disease POC |
Oncology |
Blood Collection Tubes |
|
Main Products |
Diagnostic reagents for: Cholesterol, Neutral Lipids, and Syphilis |
Instruments & diagnostic reagents for coagulation & fibrinolysis |
Diagnostic reagents for HbA1c |
Diagnostic reagents for Influenza and Adenovirus |
Diagnostic reagents for Liver Cancer |
Plastic high-speed-clotting vacuum blood collection tubes. |
(2) Diagnostic Reagents (Western)
The diagnostic business in the E.U. and the U.S. will be expanded through America-based Sekisui Diagnostics, LLC., implementing the following two measures to increase profitability:
Structural Reform: The current 9 business sites will be considered for optimization by 2018.
Expansion with a major corporation: As coagulation is an important field, we aim to expand sales through closer ties with one of the major corporations for the coagulation reagents & instruments.
(3) Pharmaceutical and Fine Chemical business
The manufacturing, under contract, of various Active Pharmaceutical Ingredients and intermediates currently takes place at the Sekisui Medical plant in Iwate (Hachimantai City), our aim is to expand the plant with a new wing near 2018 in order to strengthen our pharmaceutical business.
2) Expanding Frontiers
We are positioning China & Asia, E.U. & U.S., and New Businesses, which are expected to expand, as “frontiers” that will drive the growth of SMD in future, as well as strengthening Market Development, and R&D.
(1) China, Asia
Currently we are developing our Diagnostics Business with Sekisui Medical Technology (China) Co., Ltd (HQ: Beijing, Branch Office: Shanghai), our current foothold of Diagnostic Business in China. A diagnostic reagent manufacturing & sales company was established in December last year due to the rapid increase in demand in recent years. The new company aims to commence production in the first half of the 2017 fiscal year and through this accelerate business development in China.
The market is also expected to expand in emerging countries such as India & South East Asia, in accordance with their economic development. Sekisui Chemical Group will make use of its sales bases in each region and promote market exploration going forward.
|
<Newly-established Chinese Company> |
|
|
・Name: |
Sekisui Medical Technology (Suzhou) CO., LTD |
|
・Address: |
Plant6, Emerging Industry Industrial Workshop, 78th Xinglin Street, Suzhou Industrial Park, Suzhou, P.R. China. |
|
・Est.: |
December 21st, 2015 (launch scheduled for first half of 2017 FY.) |
|
・Size: |
4500m2 floor area |
|
・Capital: |
$5 million USD |
|
・Primary Business:Clinical Diagnostic Reagent production, procurement, and sales. Instrument procurement and sales. |
|
(2) Western
In European and U.S. markets we will continue to expand with the introduction of our diagnostic reagents and analyzers, which have proven to be highly competitive in Japan. In order to achieve this we plan on expanding and strengthening our sales network through further merging and acquisition activities
Although we are currently expanding our Drug Development Solutions in the U.S. & Europe, with the U.S.-based Sekisui Xenotech LLC., as our foothold, we aim to expand this business even further by improving the capabilities of our in-vitro testing services and strengthening ties with the Sekisui Medical Japanese Base.
(3) New Business
As part of our aim to expand into new fields, we will attempt to create new operations in new areas through the cooperation among medical related subsidiaries and among medical business divisions.
|
・Entry into the Clinical (human) field through the Drug Development Solutions Business |
|
・Full-scale entry into the pharmaceutical peptide business |
|
・Expansion of the enzyme business for pharmaceutical & diagnostic reagents |
(Reference: The history of Medical business in recent years of Sekisui Chemical Group) |
|
October 2006: Acquired Daiichi Pure Chemicals Co., Ltd. from Daiichi Pharmaceutical Co., Ltd |
|
April 2008: Sekisui Chemical’s Medical Product Division was merged with Daiichi Pure Chemicals Co., Ltd., and the company name was changed to Sekisui Medical Co., Ltd. |
|
August 2008: Acquired the US-based in vitro pre-clinical studies contract research organization, XenoTech, LLC. |
|
April 2009: Acquired the US-based diagnostics business company, American Diagnostica Inc. |
|
February 2010: Established Sekisui Medical Technology (China) Ltd. |
|
February 2011: Acquired the diagnostic business from US-based Genzyme Corporation, established Sekisui Diagnostics.LLC in the United States and also started business in the UK, Canada and Germany. |
|
April 2012: Merged America Diagnostica Inc. with Sekisui Diagnostics, LLC. |
|
December 2015: Acquired Eidia Co., Ltd from Eisai Co., Ltd. |
|
December 2015: Established Sekisui Medical Technology (Suzhou) Co., Ltd. |
Disclaimer
This press release may contain forward-looking statements. Such forward-looking statements are based on current expectations and beliefs and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements due to changes in global economic, business, competitive market and regulatory factors.