SEKISUI MEDICAL CO., LTD. (President: Mutsumi Fukuda, hereinafter Sekisui Medical), a 100% subsidiary of SEKISUI CHEMICAL, CO., LTD. (President: Naofumi Negishi, hereinafter Sekisui Chemical), has signed an OEM agreement to supply blood coagulation analyzers and dedicated reagents to Nihon Kohden Corporation (President & COO: Fumio Suzuki, hereinafter Nihon Kohden) in order to expand its overseas business in the field of blood coagulation analysis.
| 1. | Background Sekisui Medical entered the field of blood coagulation analysis reagents in 1979 with “Testzym”, a series of reagents based on the synthetic substrate method. Thanks to its product lineup of Nanopia and Coagpia brands, it now holds the largest share of domestic sales in the field (internal estimation). Sekisui Medical released the blood coagulation analyzer “Coapresta 2000” in March 2007 and began its medical instrument business where instruments and reagents are combined. Over 200 units of “Coapresta 2000” have been installed mainly in large hospitals such as university hospitals, and it holds the top share for annual domestic deliveries of instruments in its class (internal estimation). Moreover, in April 2009 Sekisui Chemical acquired the US-based American Diagnostica Inc. (ADI), a company that has proven itself in the field of blood coagulation analysis reagents. Joint development of blood coagulation analysis reagents with ADI will create a structure to further enhance Sekisui Medical’s product lineup and is an example of the efforts being made to expand the instruments business in the field of blood coagulation analysis. With this agreement with Nihon Kohden, Sekisui Medical is going to expand the overseas business of blood coagulation analysis instruments and reagents through Nihon Kohden’s global sales and service network. <About Nihon Kohden> Nihon Kohden is a major manufacturer of medical equipment, and it is the sole domestic producer of the electroencephalograph (EEG) and AED machines that hold top market share in the world. In the field of clinical lab equipment, it does business domestically and internationally with its hematology analyzer brand “Celltac”. The present agreement will allow Nihon Kohden to offer a total system that includes hematology analyzers to hematology laboratories in order to expand its sales in the hematology business. |
|||
|
|
|
|||
| 2. | Contract details
|
[References]
1. Terms
| 1) | Blood coagulation analysis The analysis examines the concentration, functionality and activity of blood coagulation factors contained in plasma obtained by centrifuging blood. In a hematology laboratory in a large hospital, blood is tested using a blood coagulation analyzer and a hematology analyzer. The lineup of the two instruments makes it possible to offer a total system to hematology laboratories. |
|
|
|
| 2) | Hematology analyzer This instrument measures hematology parameters such as the red blood cell count and the white blood cell count in blood, which are utilized in the diagnosis of anemia, bacterial infection, and so on. |
2. Product Descriptions
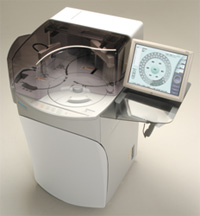
| 1) | Blood coagulation analyzer “Coapresta 2000” This is a fully automated blood coagulation analyzer that analyzes blood coagulation, which requires rapid processing after blood collection, using simple operations with real-time parameter selection. It is manufactured by Shimadzu Corporation. |
|
|
|
| 2) | “Testzym” This is a brand name for blood coagulation analysis reagents based on the synthetic chromogenic substrate method. The activity value of the analyte can be obtained by colorimetric quantitation of the chromogenic substance derived from degradation of the synthetic substrate. |
|
|
|
| 3) | “Nanopia” This is a brand name for blood coagulation analysis reagents to measure D-dimer by latex agglutination. Blood coagulation is a phenomenon which is started from platelet aggregation and in which fibrinogen turns into fibrin. Fibrin is dissolved by an enzyme plasmin. This phenomenon is referred to as fibrinolysis, and the substances degraded here are fibrin degradation products, one of which is called D-dimer. D-dimer can be used as an indicator to reflect the state of fibrinolysis. |
|
|
|
| 4) | “Coagpia” This is a brand name for blood coagulation analysis reagents used for analyzing factors including prothrombin time (PT), APTT(activated partial thromboplastin time),and plasma fibrinogen. |
3. Company overview
| 1) | SEKISUI MEDICAL CO., LTD.
|
||||||||||||||||
|
|
|
||||||||||||||||
| 2) | Nihon Kohden Corporation
|
||||||||||||||||
|
|
|
||||||||||||||||
| 3) | American Diagnostica Inc. (ADI)
|
Disclaimer
This press release may contain forward-looking statements. Such forward-looking statements are based on current expectations and beliefs and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements due to changes in global economic, business, competitive market and regulatory factors.
This press release may contain forward-looking statements. Such forward-looking statements are based on current expectations and beliefs and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements due to changes in global economic, business, competitive market and regulatory factors.